Industrial Laser Marking Systems / Laser Markers
8 Types of Common Lasers
-
Tags:
- Automotive , Medical , Semiconductor
Dating back to 1960, when Theodore Maiman invented the first laser, this form of light has evolved into a tool used across industries. From medical treatment to manufacturing processing, the power of lasers is used across a myriad of industries and for many types of applications.
When most think of lasers, the ubiquitous red light lasers often come to mind. However, there are many different types of lasers. In fact, there are over eight types of lasers across three categories! In this blog, we'll explore each category of laser, what the category entails, uses for lasers, and eight different types of laser machines that make up these categories.
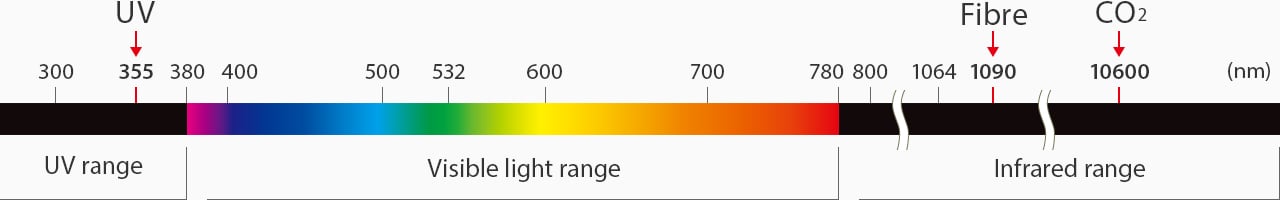
Light wavelength distribution map
Solid-State Lasers
Solid-state lasers are types of lasers that use a solid material as its gain medium. The most common solid-state laser media are yttrium aluminium garnet (YAG) and yttrium vanadate (YVO4). When a laser diode shines light on the doped material, the neodymium atoms are excited and emit light. This light is then amplified by the material and emitted as a laser beam.
YVO4 requires large amounts of energy to produce a stable high-output energy amount. This medium has recently taken over in popularity compared to YAG mediums. It's known for assisting in the high-quality marking of metals, plastic, and ceramic.
YAG mediums require less energy than YVO4 lasers, which is why they reigned popular as the universal marking structure for so long. However, because of their need for less energy, this structure is not as stable as YVO4. YAG is known for fine marking and processing silicon wafers, plastics, and reflective materials.
Within the solid-state laser category are three different types of lasers: Green, UV, and Fibre.
Green Laser
Green lasers, also known as second-harmonic lasers, are notable for their specialised ability to process at a micron level. The laser begins at a 1064 nm wavelength and then passes through a nonlinear crystal for conversion into a 532 nm wavelength. With the much shorter wavelength that appears green to the human eye, intricacy is a green laser's special power. Green lasers are used as pointing devices and for micron-level marking and processing.
UV Laser
UV lasers, also known as third-harmonic lasers, are a specialised type of solid-state laser that uses “cold marking.” Cold marking refers to a UV laser's strength of absorption that produces very little heat when marking. These lasers start at a 1064 nm wavelength and then pass through two nonlinear crystals to transition to a 355 nm wavelength.
Because of the extremely small beam, small processing and minimal damage are two key components of a UV laser. UV lasers are powerful enough to create high contrast marks on highly reflective materials like copper, gold, and silver.
Fibre Laser
Solid-state fibre lasers are highly regarded for their high average power, fast speed, and excellent cooling efficiency. With the ability to mark on metal, plastic, and ceramic with a 1030-2100 nm wavelength, it's even more impressive that fibre lasers can engrave, anneal, mark, etch, cut, and remove burrs. Fibre lasers have the smallest marking unit size, making them the easiest to retrofit, or integrate in production settings with limited space.
Gas Lasers
Gas lasers are used for machining and marking applications like marking labels, etching plastic and resins, processing, and cutting. These laser types use gas as the medium instead of a solid or liquid. The four types of gas lasers are CO₂, He-Ne, excimer, and argon.
CO₂ Laser
CO₂ gas lasers have the longest wavelength compared to fibre, YAG, and YVO4. CO₂ lasers have a wavelength of 10600 nm. Because of the long wavelength, there is more heat transfer than other lasers. Moreover, CO₂ lasers are commonly used for cutting materials.
He-Ne Laser
He-Ne gas lasers have a wavelength of 630 nm and appear red to the human eye. He-Ne lasers are commonly known for their use as pointing devices in classrooms or during presentations. These laser types are also used in store barcode scanners and measurement devices. Unlike many other different types of lasers, He-Ne lasers do not do manufacturing processing.
Excimer Laser
Excimer gas lasers use inert gas and hydrogen gas to produce a short UV wavelength of 193 nm. These lasers are commonly used for dermatology and optometry treatments. In fact, excimer lasers are proven to treat skin conditions like alopecia, psoriasis, leukoderma, and more. These lasers are also used in optometry to vaporise the lens of human eyes.
Argon Laser
Similar to excimer lasers, argon gas lasers are also used for various health conditions, scientific applications, and biomedical research. Argon lasers use a 488 to 514 nm wavelength, and one popular type of argon laser treatment is for glaucoma or diabetic eye disease treatment.
Liquid Lasers
Dye Laser
Dye lasers are liquid lasers with a wavelength of 330 to 1300 nm commonly used for scientific applications. These lasers use organic liquid dyes to power their wavelengths and produce fluorescent light.
3-Axis CO₂ Laser Marker ML-Z9500

World’s first 3-Axis control laser marker
3-Axis Hybrid Laser Marker MD-X2000/X2500

World's first laser marker with full-field auto-focus
We’re here to provide you with more details.
Reach out today!
KEYENCE's Lasers
At KEYENCE, we specialise in lasers made for manufacturing processing.
KEYENCE's CO₂ Laser: 3-Axis CO₂ Laser Marker ML-Z Series
KEYENCE's ML-Z Series is a versatile machine tackling marking, drilling, and decapsulation on materials like paper, wood, rubber, ceramics, and glass. The ML-Z is also equipped with 3-Axis control and multiple beam options for refined processing.
Get detailed information on our products by downloading our catalogue.
View Catalogue
Interested in Learning More About Lasers?
Lasers are unique and versatile equipment powered by either solids, gas, or liquid. Each laser type specialises in solutions for manufacturers, scientists, and other professional applications. There is so much more to learn about green, UV, fibre lasers, CO₂, He-Ne, excimer, argon, and dye lasers that assist you in incorporating lasers into your operations. At KEYENCE, we are laser experts and welcome any questions at any time. Ask KEYENCE, and our knowledgeable team will get back to you shortly!
We’re here to provide you with more details.
Reach out today!





